There are two primary classes of molecules, which are polar and nonpolar. There are some molecules that are clearly one or the other, but there are others that have some polarity and fall in the middle. If you understand certain characteristics of polar and nonpolar molecules, you will be able to predict whether a molecule is one or the other much more easily.
In chemistry, polarity is the distribution of electric charge around molecules, atoms, or chemical groups. You will have a polar molecule when there is an electronegativity difference between the bonded atoms, whereas nonpolar molecules have electrons that are shared equally between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. Read on to learn how to tell if a compound is polar.
Do the Atoms Share Electrons Equally in a Covalent Bond?
When you have a polar molecule, two atoms do not share electrons equally in a covalent bond. As a result, a dipole forms so that one part of the molecule has a slightly positive charge and the other part carries a slightly negative charge. There is usually a difference between the electronegativity of each atom. If it is a small difference, you will have a polar covalent bond. If the difference is too large, you end up with an ionic bond.
Look Them up
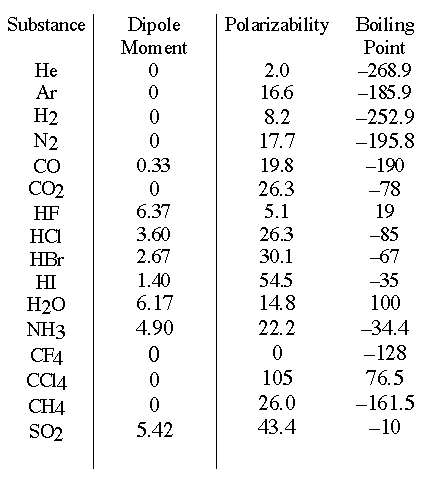
You can look the atoms up on a table to determine which type of bond they will form; if the electronegativity between the two atoms is between 0.5 and 2.0, they will form a polar covalent bond. If the electronegativity difference between the atoms is greater than 2.0, then the bond will be ionic. Note that Ionic compounds are extremely polar molecules. Examples include water (H2O), Ammonia (NH3), and Sulfur dioxide (SO2).
If the two atoms have an electronegativity difference of less than 0.5, then the bond is nonpolar. In addition, nonpolar molecules form when the electric charges of two atoms cancel each other out. Examples of nonpolar molecules include carbon dioxide (CO2), Methane (CH4), and Ethylene (C2H4).
Draw the Lewis Structure of the Molecule
When you draw the Lewis structure of the molecule, you can analyze its shape. There are five possible categories of shapes, which include linear, tetrahedral, trigonal planar, bent, and trigonal pyramid. The first three are symmetric shapes, and the last two are asymmetric. Polar molecules are not symmetric, so they will fall into one of the last two categories. Once you know what category your molecule belongs to, you can determine if it is polar or nonpolar.
Almost all bent molecules are polar, but there are a few exceptions. The C-H bond is a great example of a molecule that is nonpolar, even though the bonds are slightly polar. The best thing you can do is memorize any exceptions because there are only a few.
When you learn about polar and nonpolar molecules, there are some basic rules that you should remember. The polar molecules have positive and negative charged ends, whereas nonpolar molecules do not. The distribution of electrons is equal in nonpolar molecules, so they will be symmetric. You should note that most of the hydrocarbons liquids are nonpolar. In addition, you will see electrical dipole movement in polar molecules, but not in nonpolar molecules.
The first step to determining whether a molecule is polar or not is to examine its bonds. Covalent bonds share electrons, and ionic bonds give them up. Either way, they will be positively charged on one side and negatively charged on the other. Electronegativity is a measure of how strongly an element seeks electrons from another. When you examine the Periodic table, you can find the electronegativity of any of the elements. Having all of this information will give you the tools you need to determine whether a compound is polar or not.